Which Radioactive Element Is Used To Determine The Absolute Age Of Late Pleistocene Animal Remains?
As we learned in the previous lesson, index fossils and superposition are effective methods of determining the relative historic period of objects. In other words, you can use superposition to tell you that one stone layer is older than another. But determining the absolute age of a substance (its age in years) is a much greater challenge. To accomplish this, scientists use a variety of prove, from tree rings to the amounts of radioactive materials in a rock.
Lesson Objectives
- Define the difference between absolute age and relative age.
- Describe four methods of absolute dating.
- Explain what radioactivity is and give examples of radioactive decay.
- Explain how the disuse of radioactive materials helps to establish the age of an object.
- Estimate the historic period of an object, given the half-life and the amounts of radioactive and girl materials.
- Give four examples of radioactive materials that are used to date objects, and explicate how each is used.
Tree Rings
In regions exterior the tropics, trees grow more than quickly during the warm summer months than during the libation winter. This design of growth results in alternating bands of lite-colored, low density "early on wood" and dark, high density "late wood". Each night band represents a winter; by counting rings it is possible to detect the age of the tree (Figure 11.22). The width of a series of growth rings can give clues to past climates and various disruptions such as forest fires. Droughts and other variations in the climate make the tree grow slower or faster than normal, which shows up in the widths of the tree rings. These tree ring variations will appear in all trees growing in a certain region, so scientists can match up the growth rings of living and expressionless trees. Using logs recovered from one-time buildings and ancient ruins, scientists have been able to compare tree rings to create a continuous record of tree rings over the past ii,000 years. This tree band record has proven extremely useful in creating a record of climate change, and in finding the historic period of ancient structures.

Figure 11.22: Cross-department showing growth rings. The thick, light-colored part of each ring represents rapid spring and summer growth. The thin, nighttime function of each ring represents slow autumn and winter growth.
Ice Cores and Varves
Several other processes result in the accumulation of distinct yearly layers that tin can exist used for dating. For case, layers class within glaciers considering there tends to exist less snow in the summertime, assuasive a dark layer of dust to accumulate on meridian of the winter snowfall (Figure 11.23). To study these patterns, scientists drill deep into ice sheets, producing cores hundreds of meters long. Scientists clarify these ice cores to determine how the climate has changed over fourth dimension, likewise as to measure out concentrations of atmospheric gases. The longest cores have helped to grade a record of polar climate stretching hundreds of thousands of years back.

Figure eleven.23: Water ice core department showing annual layers.
Another example of yearly layers is the degradation of sediments in lakes, particularly the lakes that are located at the end of glaciers. Rapid melting of the glacier in the summer results in a thick, sandy deposit of sediment. These thick layers alternate with sparse, clay-rich layers deposited during the winter. The resulting layers, calledvarves, give scientists clues about by climate conditions. For instance, an especially warm summertime might outcome in a very thick layer of sediment deposited from the melting glacier. Thinner varves can betoken colder summers, because the glacier doesn't cook every bit much and carry as much sediment into the lake.
Age of Earth

Figure xi.24: Lord Kelvin.
While tree rings and other annual layers are useful for dating relatively recent events, they are not of much use on the vast scale of geologic time. During the 18th and 19th centuries, geologists tried to approximate the age of Earth with indirect techniques. For example, geologists measured how fast streams deposited sediment, in society to effort to calculate how long the stream had been in existence. Not surprisingly, these methods resulted in wildly different estimates, from a few one thousand thousand years to "quadrillions of years". Probably the most reliable of these estimates was produced past the British geologist Charles Lyell, who estimated that 240 meg years have passed since the appearance of the beginning animals with shells. Today scientists know his estimate was too young; we know that this occurred about 530 million years agone.
In 1892, William Thomson (later known as Lord Kelvin) calculated the historic period of Earth in a systematic fashion (Figure 11.24). He assumed that the Earth began every bit a ball of molten rock, which has steadily cooled over fourth dimension. From these assumptions, he calculated that the Earth was 100 million years onetime. This estimate was a blow to geologists and supporters of Charles Darwin'due south theory of evolution, which required an older Earth to provide time for evolution to take identify.
Thomson'south calculations, however, were soon shown to be flawed when radioactivity was discovered in 1896.Radioactivity is the tendency of certain atoms to decay into lighter atoms, emitting energy in the process. Radioactive materials in World's interior provide a steady source of estrus. Calculations of Earth'south age using radioactivity showed that Earth is actually much older than Thomson calculated.
Radioactivity
The discovery of radioactive materials did more than than disprove Thomson'south estimate of Earth'due south age. It provided a way to find the absolute age of a rock. To understand how this is washed, it is necessary to review some facts about atoms.
Atoms contain iii particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, while electrons orbit around the nucleus. The number of protons determines which chemical element you're examining. For example, all atoms of carbon have six protons, all atoms of oxygen take eight protons, and all atoms of gold accept 79 protons. The number of neutrons, however, is variable. An atom of an element with a different number of neutrons is anisotope of that chemical element. For example, the isotope carbon-12 contains six neutrons in its nucleus, while the isotope carbon-13 has 7 neutrons.
Some isotopes areradioactive, which means they are unstable and likely to decay. This means the cantlet will spontaneously change from an unstable form to a stable form. There are two forms of nuclear disuse that are relevant in how geologists can date rocks (Table (eleven.one):
| Particle | Limerick | Issue on Nucleus |
|---|---|---|
| Blastoff | ii protons, ii neutrons | The nucleus contains two fewer protons and two fewer neutrons. |
| Beta | 1 electron | One neutron decays to form a proton and an electron, which is emitted. |
If an element decays by losing an blastoff particle, it will lose two protons and 2 neutrons. If an atom decays by losing a beta particle, it loses just ane electron.
So what does this have to do with the age of Earth? Radioactive decay eventually results in the formation of stabledaughter products. Radioactive materials disuse at known rates. As time passes, the proportion of radioactive isotopes volition decrease and the proportion of daughter isotopes volition increase. A stone with a relatively high proportion of radioactive isotopes is probably very young, while a rock with a high proportion of girl products is probably very one-time.
Scientists measure the rate of radioactive disuse with a unit calledhalf-life. The half-life of a radioactive substance is the corporeality of time, on boilerplate, it takes for half of the atoms to decay. For example, imagine a radioactive substance with a half-life of one year. When a stone is formed, it contains a sure number of radioactive atoms. Later 1 year (i half-life), half of the radioactive atoms have decayed to form stable girl products, and fifty% of the radioactive atoms remain. After some other yr (two one-half-lives), half of the remaining radioactive atoms have decayed, and 25% of the radioactive atoms remain. After the third year (three half-lives), 12.5% of the radioactive atoms remain. Afterwards four years (4 half-lives), half-dozen.25% of the radioactive atoms remain, and after 5 years (v half-lives), only 3.125% of the radioactive atoms remain.
If you notice a stone whose radioactive cloth has a half life of i year and measure 3.125% radioactive atoms and 96.875% daughter atoms, you tin assume that the substance is 5 years quondam. The disuse of radioactive materials can be shown with a graph (Figure xi.25). If you find a rock with 75% of the radioactive atoms remaining, well-nigh how old is it?

Figure 11.25: Decay of an imaginary radioactive substance with a half-life of 1 year.
Radiometric Dating of Rocks
In the process ofradiometric dating, several isotopes are used to date rocks and other materials. Using several different isotopes helps scientists to check the accuracy of the ages that they calculate.
Carbon Dating
Earth's atmosphere contains three isotopes of carbon. Carbon-12 is stable and accounts for 98.9% of atmospheric carbon. Carbon-13 is also stable and accounts for 1.i% of atmospheric carbon. Carbon-xiv is radioactive and is plant in tiny amounts. Carbon-14 is produced naturally in the atmosphere when cosmic rays collaborate with nitrogen atoms. The amount of carbon-14 produced in the atmosphere at whatever item time has been relatively stable through time.
Radioactive carbon-14 decays to stable nitrogen-14 by releasing a beta particle. The nitrogen atoms are lost to the atmosphere, but the amount of carbon-14 decay tin can be estimated by measuring the proportion of radioactive carbon-14 to stable carbon-12. Every bit a substance ages, the relative amount of carbon-xiv decreases.
Carbon is removed from the atmosphere by plants during the process of photosynthesis. Animals swallow this carbon when they eat plants or other animals that have eaten plants. Therefore carbon-xiv dating can be used to date found and animal remains. Examples include timbers from an old building, bones, or ashes from a fire pit. Carbon dating tin can be effectively used to find the age of materials between 100 and 50,000 years former.
Potassium-Argon Dating
Potassium-twoscore decays to argon-xl with a half-life of 1.26 billion years. Because argon is a gas, information technology can escape from molten magma or lava. Therefore any argon that is constitute in a crystal probably formed as a consequence of the decay of potassium-40. Measuring the ratio of potassium-40 to argon-40 will yield a proficient judge of the age of the sample.
Potassium is a common element found in many minerals such as feldspar, mica, and amphibole. The technique can be used to appointment igneous rocks from 100,000 years to over a billion years old. Considering it tin be used to date geologically immature materials, the technique has been useful in estimating the historic period of deposits containing the basic of human ancestors.
Uranium-Lead Dating
Two isotopes of uranium are used for radiometric dating. Uranium-238 decays to form lead-206 with a half-life of 4.47 billion years. Uranium-235 decays to course atomic number 82-207 with a half-life of 704 meg years.
Uranium-atomic number 82 dating is normally performed on crystals of the mineral zircon (Figure 11.26). When zircon forms in an igneous rock, the crystals readily accept atoms of uranium but reject atoms of atomic number 82. Therefore, if whatsoever pb is found in a zircon crystal, it can be causeless that it was produced from the decay of uranium.

Effigy eleven.26: Zircon crystal.
Uranium-atomic number 82 dating tin exist used to date igneous rocks from 1 meg years to around iv.5 billion years quondam. Some of the oldest rocks on World have been dated using this method, including zircon crystals from Australia that are iv.4 billion years onetime.
Limitations of Radiometric Dating
Radiometric dating tin only be used on materials that contain measurable amounts of radioactive materials and their daughter products. This includes organic remains (which compared to rocks are relatively immature, less than 100,000 years old) and older rocks. Ideally, several unlike radiometric techniques will be used to date the same rock. Agreement between these values indicates that the calculated historic period is accurate.
In general, radiometric dating works all-time for igneous rocks and is non very useful for determining the age of sedimentary rocks. To estimate the historic period of a sedimentary rock deposit, geologists search for nearby or interlayered igneous rocks that can be dated. For instance, if a sedimentary rock layer is sandwiched between two layers of volcanic ash, its age is between the ages of the ii ash layers.
Using a combination of radiometric dating, index fossils, and superposition, geologists accept synthetic a well-defined timeline of Earth history. For example, an overlying lava menstruation can give a reliable judge of the age of a sedimentary stone formation in one location. Index fossils contained in this formation can and so be matched to fossils in a different location, providing a good historic period measurement for that new rock formation equally well. As this process has been repeated all over the world, our estimates of rock and fossil ages has become more and more accurate.
Lesson Summary
Techniques such as superposition and index fossils can tell you the relative age of objects, which objects are older and which are younger. Other types of prove are needed to establish the absolute age of objects in years. Geologists utilize a variety of techniques to establish absolute age, including radiometric dating, tree rings, water ice cores, and annual sedimentary deposits called varves.
Radiometric dating is the most useful of these techniques—information technology is the only technique that can plant the age of objects older than a few g years. The concentrations of several radioactive isotopes (carbon-14, potassium-40, uranium-235 and -238) and their girl products are used to make up one's mind the age of rocks and organic remains.
Review Questions
- What four techniques are used to determine the absolute historic period of an object or upshot?
- A radioactive substance has a half-life of 5 million years. What is the age of a rock in which 25% of the original radioactive atoms remain?
- A scientist is studying a slice of textile from an ancient burying site. She determines that 40% of the original carbon-xiv atoms remain in the material. Based on the carbon decay graph (Figure eleven.27), what is the guess age of the fabric?
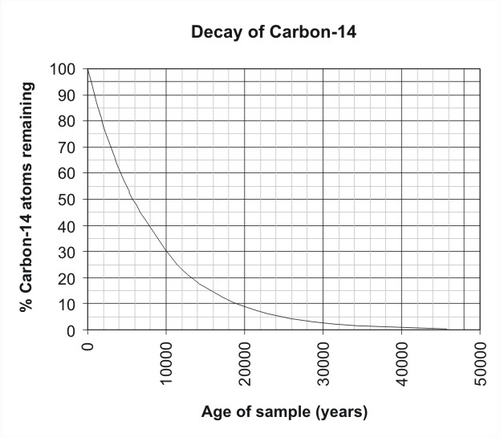
Figure 11.27: Radioactivity of Carbon-14
- Which radioactive isotope or isotopes would you employ to date each of the following objects? Explain each of your choices.
- A four billion year old piece of granite.
- A one million year one-time bed of volcanic ash that contains the footprints of hominids (human ancestors).
- The fur of a woolly mammoth that was recently recovered frozen in a glacier.
- A fossilized trilobite recovered from a bed of sandstone that is about 500 1000000 years old.
- The principle of uniformitarionism states that the present is the key to the past. In other words, the processes that we encounter happening today probably worked in a like style in the past. Why is it important to presume that the rate of radioactive decay has remained constant over time?
Vocabulary
- absolute age
- The historic period of an object in years.
- alpha particle
- Particle consisting of 2 protons and two neutrons that is ejected from the nucleus during radioactivity.
- beta particle
- Particle consisting of a single electron that is ejected from the nucleus during radioactive decay. A beta particle is created when a neutron decays to form a proton and the emitted electron.
- girl product
- Stable substance that is produced past the decay of a radioactive substance. For instance, uranium-238 decays to produce atomic number 82-207.
- half-life
- Amount of time required for half of the atoms of a radioactive substance to decay and form daughter products.
- ice core
- Cylinder of ice extracted from a glacier or water ice sheet.
- radioactive
- Substance that is unstable and likely to emit energetic particles and radiation.
- radioactive decay
- Emission of high-free energy particles and/or radiation by certain unstable atoms.
- radiometric dating
- Process of using the concentrations of radioactive substances and daughter products to estimate the age of a material. As substances age, the amounts of radioactive atoms decrease while the amounts of daughter materials increase.
- tree ring
- Layer of woods in a tree that forms in one year. You tin determine the age of a tree by counting its rings.
- varve
- Thin layer of sediment deposited on a lakebed over the course of one yr ordinarily found at the bottom of glacial lakes.
Points to Consider
- Why are techniques like tree rings, ice cores, and varves only useful for events that occurred in the final few thousand years?
- Why was information technology then important for Darwin and his followers to evidence that the Earth was very old?
- Why is it important to use more than one method to find the age of a rock or other object?
Source: https://courses.lumenlearning.com/earthscience/chapter/absolute-ages-of-rocks/
Posted by: nashtheken.blogspot.com

0 Response to "Which Radioactive Element Is Used To Determine The Absolute Age Of Late Pleistocene Animal Remains?"
Post a Comment